
Chemistry, 21.10.2019 17:40 allieb12334
If 143 grams of chromium react with an excess of oxygen, as shown in the balanced chemical equation below, how many grams of chromium oxide can be formed? show all your work for the calculations for full credit.
4cr + 3o2 yields 2cr2o3

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:10
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1


Chemistry, 22.06.2019 22:30
Write and balance the chemical equation that represents the reaction of aqueous sulfuric acid with aqueous sodium hydroxide to form water and sodium sulfate. include phases.
Answers: 1

You know the right answer?
If 143 grams of chromium react with an excess of oxygen, as shown in the balanced chemical equation...
Questions

English, 18.03.2021 01:20

English, 18.03.2021 01:20

Mathematics, 18.03.2021 01:20





Mathematics, 18.03.2021 01:20




Computers and Technology, 18.03.2021 01:20



Mathematics, 18.03.2021 01:20


Mathematics, 18.03.2021 01:20



Biology, 18.03.2021 01:20

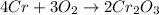
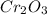 = 152g/mol
= 152g/mol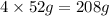 of Chromium produces
of Chromium produces 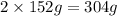 of
of 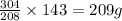 of Chromium oxide(
of Chromium oxide(


