
Chemistry, 12.04.2021 08:20 shadoris26
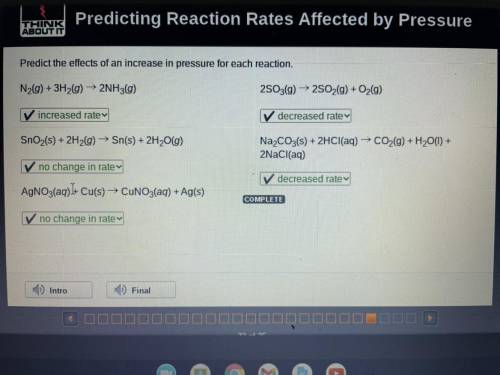
Predict the effects of an increase in pressure for each reaction.
N2(g) + 3H2(g) → 2NH3(g) 2S03(g) ► 2S02(g) + O2(g)
SnO2(s) + 2H2(g) → Sn(s) + 2H20(g)
Na2CO3(s) + 2HCl(aq) → CO2(g) + H20(1) +
2NaCl(aq)
AgNO3(aq))+ Cu(s) → CUNO3(aq) + Ag(s)
COMPLETE

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Measuring which physical property is most likely to produce the most precise results when trying to identify a substance
Answers: 1

Chemistry, 22.06.2019 07:00
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2

Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1
You know the right answer?
Predict the effects of an increase in pressure for each reaction.
N2(g) + 3H2(g) → 2NH3(g) 2S03(g)...
Questions

Biology, 09.07.2021 23:50








History, 10.07.2021 01:00




Mathematics, 10.07.2021 01:00









