
Chemistry, 12.04.2021 14:00 Knownothing
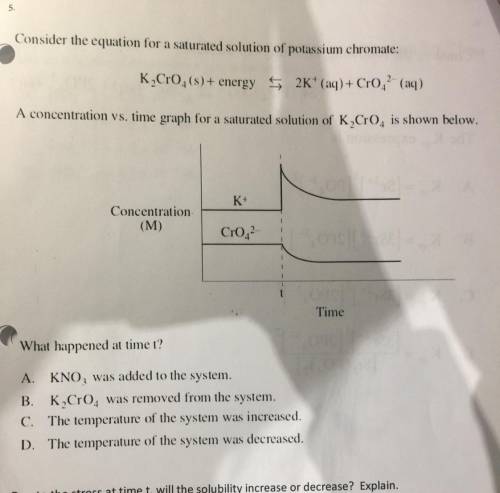
5.
Consider the equation for a saturated solution of potassium chromate:
K, CrO4(s)+ energy 5 2K+ (aq) + Cro. (aq)
A concentration vs. time graph for a saturated solution of K Cr0is shown below.
K+
Concentration
(M)
Cr02
Time
What happened at time t?
A. KNO, was added to the system.
B. K Cro. was removed from the system.
C. The temperature of the system was increased.
D. The temperature of the system was decreased.
Due to the stress at time t, will the solubility increase or decrease? Explain.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3

Chemistry, 22.06.2019 22:30
Akno3 solution containing 51 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 3

You know the right answer?
5.
Consider the equation for a saturated solution of potassium chromate:
K, CrO4(s)+ energy 5...
K, CrO4(s)+ energy 5...
Questions

Mathematics, 02.10.2019 01:50

Biology, 02.10.2019 01:50


English, 02.10.2019 01:50


English, 02.10.2019 01:50



Mathematics, 02.10.2019 01:50

Chemistry, 02.10.2019 01:50

Geography, 02.10.2019 01:50



English, 02.10.2019 01:50

Mathematics, 02.10.2019 01:50

Computers and Technology, 02.10.2019 01:50



Mathematics, 02.10.2019 02:00



