
Chemistry, 12.04.2021 18:10 jameskarbar9p8c9d2
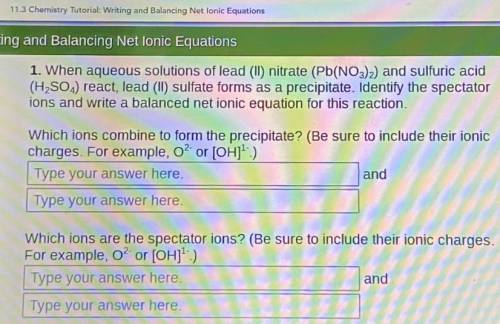
When aqueous solutions of lead (II) nitrate (Pb(NO3)2) and sulfuric acid (H, SO.,) react, lead (II) sulfate forms as a precipitate. Identify the spectator ions and write a balanced net ionic equation for this reaction.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1

Chemistry, 22.06.2019 19:50
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3

Chemistry, 22.06.2019 21:50
Given the data below for the reaction, 2 a + 2 b + 4 c => d + e + 3 f, the reaction is fill in the [ ] order in a, fill in the [ ] order in b, fill in the [ ] order in c and fill in the [ ] order overall. (use the words "first, second, third, fourth" to fill each blank)experimentinitial conc of a, mol/l initial conc of b, mol/l initial conc of c, mol/l initial rate, mol/l.s1 0.1 0.1 0.2 2 x 10-32 0.2 0.3 0.2 6 x 10-33 0.3 0.1 0.2 2 x 10-34 0.4 0.3 0.4 1.2 x 10-2
Answers: 2

Chemistry, 22.06.2019 22:10
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1
You know the right answer?
When aqueous solutions of lead (II) nitrate (Pb(NO3)2) and sulfuric acid (H, SO.,) react, lead (II)...
Questions


Arts, 12.11.2020 23:30

History, 12.11.2020 23:30

History, 12.11.2020 23:30


Business, 12.11.2020 23:30

Geography, 12.11.2020 23:30



Mathematics, 12.11.2020 23:30


Mathematics, 12.11.2020 23:30





Law, 12.11.2020 23:30





