ASAP PLS
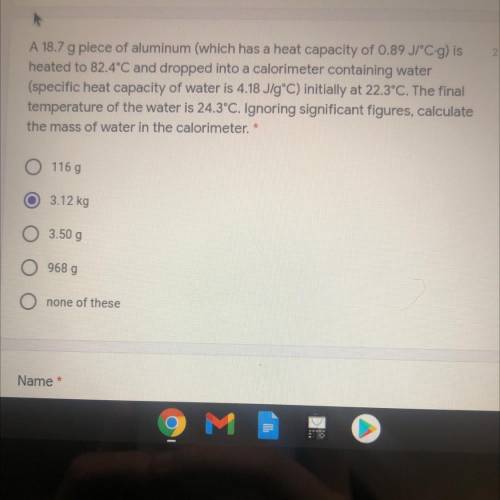
A 18.7 g piece of aluminum (which has a heat capacity of 0.89 JPC-g) is
heated to 82...

ASAP PLS
A 18.7 g piece of aluminum (which has a heat capacity of 0.89 JPC-g) is
heated to 82.4°C and dropped into a calorimeter containing water
(specific heat capacity of water is 4.18 J/gºC) initially at 22.3°C. The final
temperature of the water is 24.3°C. Ignoring significant figures, calculate
the mass of water in the calorimeter. *

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
The boiling point of liquids is very high what does it indicate
Answers: 1


Chemistry, 22.06.2019 18:50
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1

Chemistry, 22.06.2019 22:30
Akno3 solution containing 51 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 3
You know the right answer?
Questions


Mathematics, 27.10.2020 05:20

Business, 27.10.2020 05:20

Mathematics, 27.10.2020 05:20

Arts, 27.10.2020 05:20

Mathematics, 27.10.2020 05:20

History, 27.10.2020 05:20

Computers and Technology, 27.10.2020 05:20

Physics, 27.10.2020 05:20


Social Studies, 27.10.2020 05:20


Mathematics, 27.10.2020 05:20


Social Studies, 27.10.2020 05:20


English, 27.10.2020 05:20

SAT, 27.10.2020 05:20


Arts, 27.10.2020 05:20



