
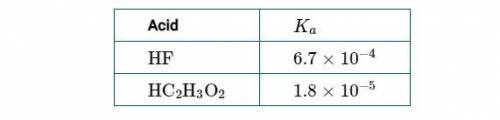
Chemistry, 12.04.2021 22:00 macylen3900
(1st Screenshot)
(a) Using the information in the table above, determine the value of ΔG° at 298K for the process represented by the equation H2O(l)⇄H2O(g).
(b) Considering your answer to part (a), indicate whether the process is thermodynamically favorable at 298K. Justify your answer.
(c) Considering your answer to part (b), explain why H2O(l) has a measurable equilibrium vapor pressure at 298K.
(2nd Screenshot)
Water vapor can be produced in two different processes, as represented below.
(d) In terms of concepts of entropy and the particle-level structure of the different phases of water, explain why the change in entropy, ΔS, is greater for process 1 than for process 2.
Please help as soon as possible


Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
The compound methyl butanoate smells like apples. its percent composition is 58.8% c, 9.9% h, and 31.4% o. what’s the empirical formula ?
Answers: 1

Chemistry, 22.06.2019 06:30
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1

Chemistry, 22.06.2019 12:00
What is the subscript for oxygen in its molecular formula
Answers: 1

Chemistry, 23.06.2019 03:30
Name atleast 3 type of energy associated with the microwave
Answers: 1
You know the right answer?
(1st Screenshot)
(a) Using the information in the table above, determine the value of ΔG° at 298K f...
Questions

Mathematics, 14.12.2020 20:20

SAT, 14.12.2020 20:20



Mathematics, 14.12.2020 20:20


Chemistry, 14.12.2020 20:20





Physics, 14.12.2020 20:20


Mathematics, 14.12.2020 20:20

English, 14.12.2020 20:20

Mathematics, 14.12.2020 20:20

Mathematics, 14.12.2020 20:20

History, 14.12.2020 20:20


Mathematics, 14.12.2020 20:20



