
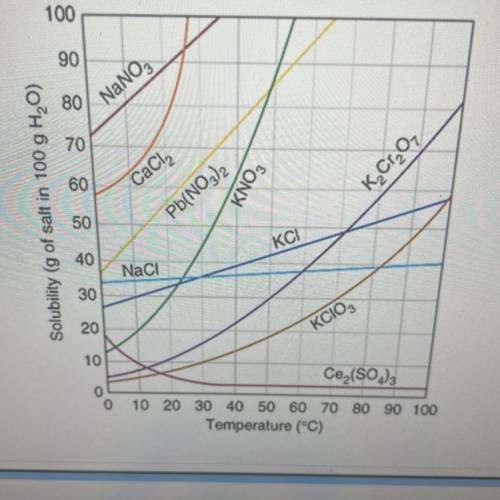
1) what type of solution (saturated or unsaturated ) is present for Pb(NO3)2 if at approximately 25 degrees c
,65 grams of the substance are present in the 100 grams of H2O
2)40 grams of KCl are dissolve in 100 grams of H2O at 10 degrees c how many grams will not dissolve
3)how many grams of H2O are needed to dissolve 50 grams of KClO3 at 70 degrees C
4)how many grams of K2Cr2O7 will dissolve in 75 grams of H2O at 90 degrees C
5) 59 grams of CaCl2 are dissolve in 100 grams of water at approximately 25 degrees c how many more grams of CaCl2 must be added to saturate the solution

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:10
Think about how you can use le chatelier’s principle to find possible solutions to the design problem. describe at least two ways to increase the yield (amount) of ammonia based on this principle.
Answers: 2

Chemistry, 22.06.2019 18:30
Which rate indicates the number of children that would be born per woman if she were to live to the end of her child bearing years
Answers: 2

Chemistry, 22.06.2019 22:00
How many moles of no2 will form when 3.3 moles of cu are reacted with excess hno3?
Answers: 3

Chemistry, 23.06.2019 02:00
What causes the appearance of lines in a emission spectrum
Answers: 1
You know the right answer?
1) what type of solution (saturated or unsaturated ) is present for Pb(NO3)2 if at approximately 25...
Questions

Chemistry, 24.09.2019 20:30

Mathematics, 24.09.2019 20:30


Biology, 24.09.2019 20:30



Social Studies, 24.09.2019 20:30


History, 24.09.2019 20:30






Computers and Technology, 24.09.2019 20:30





English, 24.09.2019 20:30



