
Chemistry, 13.04.2021 01:00 shyiann7910
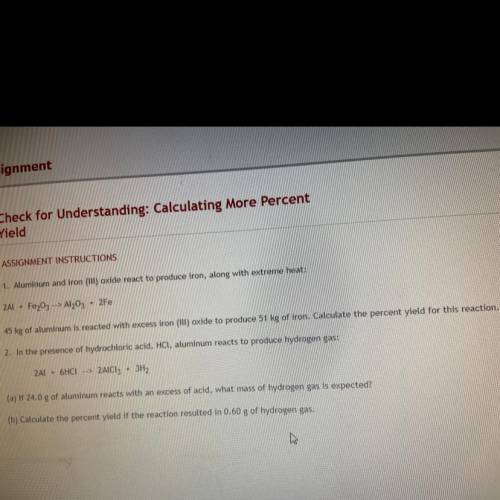
1. Aluminum and iron oxide react to produce iron along with extreme heat 2Al * Fr2O4—> Al2O3+ 2Fe 45 KG aluminum is reacted with excess iron (III) oxide to produce 51 KG of iron. Calculate the percent yield for this reaction.

Answers: 2


Another question on Chemistry



Chemistry, 22.06.2019 04:30
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3

Chemistry, 22.06.2019 07:00
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2
You know the right answer?
1. Aluminum and iron oxide react to produce iron along with extreme heat 2Al * Fr2O4—> Al2O3+ 2Fe...
Questions

Mathematics, 29.09.2019 21:30

History, 29.09.2019 21:30


Computers and Technology, 29.09.2019 21:30


Spanish, 29.09.2019 21:30



History, 29.09.2019 21:30

History, 29.09.2019 21:30



Spanish, 29.09.2019 21:30





Mathematics, 29.09.2019 21:30


Chemistry, 29.09.2019 21:30



