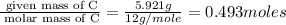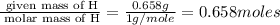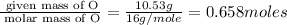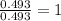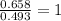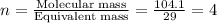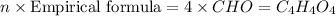A 17.11 gram sample of an organic compound containing only C, H, and O is analyzed by combustion analysis and 21.71 g CO2 and 5.926 g H2O are produced. In a separate experiment, the molar mass is found to be 104.1 g/mol. Determine the empirical formula and the molecular formula of the organic compound. Enter the elements in the order C, H, O empirical formula

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:20
Concerning the 10.0 ml of 0.50 m nacl to 100 ml of solution: when a solution is diluted, does it change the number of moles dissolved?
Answers: 3

Chemistry, 22.06.2019 16:50
Which of the following is an indication that a substance has undergone a chemical change? a. no new product has been formed. b. the color of the substance has not changed. c. the original constitute has not changed. d. the molecular structure has changed.
Answers: 1

Chemistry, 23.06.2019 01:40
Calcium carbonate decomposes at high temperatures to give calcium oxide and carbon dioxide as shown below. caco3(s) cao(s) + co2(g) the kp for this reaction is 1.16 at 800°c. a 5.00 l vessel containing 10.0 g of caco3(s) was evacuated to remove the air, sealed, and then heated to 800°c. ignoring the volume occupied by the solid, what will be the mass of the solid in the vessel once equilibrium is reached?
Answers: 1

Chemistry, 23.06.2019 03:30
Mr. rose asked his student to draw a quadrilateral with four unequal sides. an example of this kind of quadrilateral
Answers: 1
You know the right answer?
A 17.11 gram sample of an organic compound containing only C, H, and O is analyzed by combustion ana...
Questions




Mathematics, 10.07.2021 04:30




Biology, 10.07.2021 04:30

History, 10.07.2021 04:30


Advanced Placement (AP), 10.07.2021 04:30


Mathematics, 10.07.2021 04:30

English, 10.07.2021 04:30

Mathematics, 10.07.2021 04:30





Mathematics, 10.07.2021 04:30

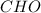 and
and 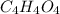 respectively.
respectively.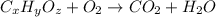
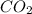 = 21.71 g
= 21.71 g
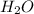 = 5.926 g
= 5.926 g
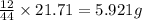 of carbon will be contained.
of carbon will be contained.
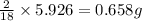 of hydrogen will be contained.
of hydrogen will be contained.