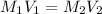Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
The diagram shows the structures of horse and cat forelimbs. what does the diagram suggest about the evolutionary relationship between these two mammals? a. they have homologous structures, indicating a common ancestor. b. they have analogous structures, indicating a common ancestor. c. they have homologous structures, indicating that they do not have a common ancestor. d. they have analogous structures, indicating that they do not have a common ancestor.
Answers: 2

Chemistry, 22.06.2019 00:00
Select the correct answer. which statement is true about a polarized object? o a. it gains electrons and becomes negatively charged. ob. it gains protons and becomes positively charged. oc. the number of positive and negative charges can be the same. od. it has to be a metal. o e. there is no change in the distribution of the charge in the object. reset next what
Answers: 3

Chemistry, 22.06.2019 05:30
What is the mass defect of a mole of nuclei with 1.8 x 10^15 j/mol binding energy?
Answers: 1

Chemistry, 22.06.2019 15:30
How does a large body of water, such as the ocean, influence climate?
Answers: 1
You know the right answer?
Mr. Whiteman has two stock solutions of sulfuric acid (H2SO4) in the supply closet: 12.1 M and 6.0 M...
Questions

History, 20.09.2020 08:01



History, 20.09.2020 08:01

English, 20.09.2020 08:01

Mathematics, 20.09.2020 08:01

Mathematics, 20.09.2020 08:01




Chemistry, 20.09.2020 08:01



Mathematics, 20.09.2020 08:01


Mathematics, 20.09.2020 08:01

Chemistry, 20.09.2020 08:01


Mathematics, 20.09.2020 08:01

History, 20.09.2020 08:01