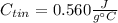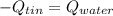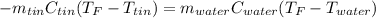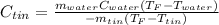Chemistry, 13.04.2021 05:10 melanyaguirre25
Solve,55.1 grams of tin at 90.6°C is dropped into a calorimeter with 75.4 grams of water at 21.0°C. The temperature of both the metal and the water reaches 27.2°C. Solve for the specific heat of tin. pleaseee once u solve it please explain how u solved it.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Write a brief passage describing a neutral atom of nitrogen-14 (n-14). describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles. use the periodic table to you. 14 protons and eletrons since its a neutral atom
Answers: 1

Chemistry, 22.06.2019 02:30
Which piece of equipment would me most useful for measuring the volume of some water? a. pan balance b. graduated cylinder c. tweezers d. flask quick
Answers: 2

Chemistry, 22.06.2019 07:10
Remember to use the proper number of significant figures and leading zeros in all calculations.gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3

Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2
You know the right answer?
Solve,55.1 grams of tin at 90.6°C is dropped into a calorimeter with 75.4 grams of water at 21.0°C....
Questions


History, 20.07.2019 03:30

Mathematics, 20.07.2019 03:30



Biology, 20.07.2019 03:30




Social Studies, 20.07.2019 03:30



Mathematics, 20.07.2019 03:30

Mathematics, 20.07.2019 03:30

English, 20.07.2019 03:30




Social Studies, 20.07.2019 03:30

Business, 20.07.2019 03:30