
The air bags in automobiles were once inflated by nitrogen gas generated by the rapid decomposition of
sodium azide, NaN3.
2 NaNz (s) 2 Na (s) + 3 N2 (g)
If an air bag has a volume of 53.4 L and is to be filled with nitrogen gas at a pressure of 1.07 atm and a
temperature of 23.7°C, how many moles of NaNz must decompose? You may assume the Ny behaves as
an ideal gas.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:50
Select the correct answer how does the heat content of the reaction change in the process of photosynthesis when a glucose molecule is formed? ca the value of is negative the value of qis positive the value of a remains constant the value of a decreases the value of equals zero e
Answers: 2


Chemistry, 22.06.2019 01:30
Asap! how do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 1

Chemistry, 22.06.2019 03:30
What is the number of moles of chemical units represented by 9.03x10^24? and how do i show work? (dumb it down )
Answers: 1
You know the right answer?
The air bags in automobiles were once inflated by nitrogen gas generated by the rapid decomposition...
Questions





English, 13.02.2020 22:48





Business, 13.02.2020 22:48

Physics, 13.02.2020 22:48






Biology, 13.02.2020 22:48



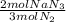 = 1.57 mol NaN₃
= 1.57 mol NaN₃

