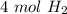Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Each pair of clay balls represents to planetesimals if each plane test molluscum pound of the same material and is separated by the same distance which pair experiences the greatest gravitational attraction
Answers: 2

Chemistry, 22.06.2019 08:30
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 22.06.2019 20:30
Citric acid has a ph between 1 and 3. it is considered to be aa. weak acidb. weak basec. strong based. strong acid
Answers: 2

Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1
You know the right answer?
2. How many moles of Hydrogen gas are needed to produce 4 moles of water?
2 H2(g) + 1 O2(g) → 2 H2O...
Questions

Mathematics, 17.05.2021 01:00



Mathematics, 17.05.2021 01:00



Mathematics, 17.05.2021 01:00

English, 17.05.2021 01:00

English, 17.05.2021 01:00


Mathematics, 17.05.2021 01:00

History, 17.05.2021 01:00


Social Studies, 17.05.2021 01:00

Social Studies, 17.05.2021 01:00


History, 17.05.2021 01:10

Chemistry, 17.05.2021 01:10

Biology, 17.05.2021 01:10

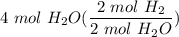 [DA] Multiply [Cancel out units]:
[DA] Multiply [Cancel out units]: