Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1

Chemistry, 22.06.2019 16:50
Ajet plane is speeding down the runway during takeoff. air resistance is not negligible. identify the forces on the jet.
Answers: 3
You know the right answer?
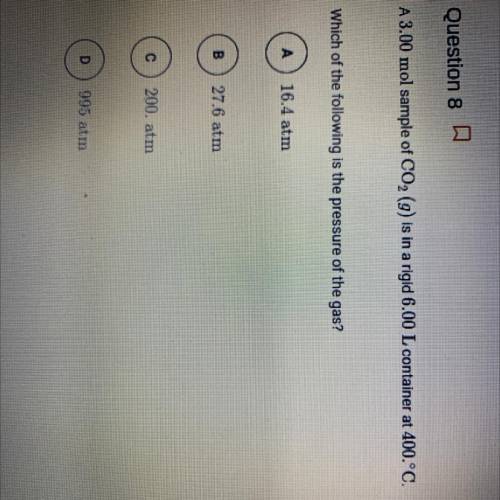
A3.00 mol sample of CO2 (g) is in a rigid 6.00 L container at 400.°C.
Which of the following is the...
Questions



Mathematics, 13.07.2019 09:00





Mathematics, 13.07.2019 09:00

Biology, 13.07.2019 09:00

Biology, 13.07.2019 09:00


History, 13.07.2019 09:00


Mathematics, 13.07.2019 09:00




English, 13.07.2019 09:00

Mathematics, 13.07.2019 09:00

Chemistry, 13.07.2019 09:00