
Chemistry, 14.04.2021 02:40 macattack6276
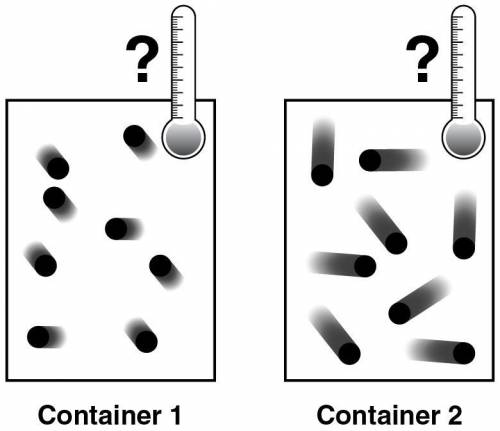
Two containers, illustrated below, are filled with the same amount and the same kind of ideal gas.
Which statement correctly describes the relationship between the contents of the two containers?
Container 1 particles have higher average kinetic energy and higher temperature than Container 2 particles.
Container 1 particles have lower average kinetic energy and lower temperature than Container 2 particles.
Container 1 particles have lower average kinetic energy and lower temperature than Container 2 particles.
Container 1 particles have lower average kinetic energy and higher temperature than Container 2 particles.
Container 1 particles have lower average kinetic energy and higher temperature than Container 2 particles.
Container 1 and Container 2 particles have the same average kinetic energy and the same temperature.
Container 1 and Container 2 particles have the same average kinetic energy and the same temperature.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2

Chemistry, 22.06.2019 21:00
One similarity and one difference between an element and a mixture of elements
Answers: 1

Chemistry, 22.06.2019 23:00
What is the mass of naoh that would have to be added to 500 ml of a solution of 0.20 m acetic acid in order to achieve a ph of 5.0?
Answers: 1
You know the right answer?
Two containers, illustrated below, are filled with the same amount and the same kind of ideal gas....
Questions

English, 16.03.2020 17:56

Biology, 16.03.2020 17:56

Mathematics, 16.03.2020 17:56


Computers and Technology, 16.03.2020 17:56








History, 16.03.2020 17:56

History, 16.03.2020 17:56



Biology, 16.03.2020 17:56





