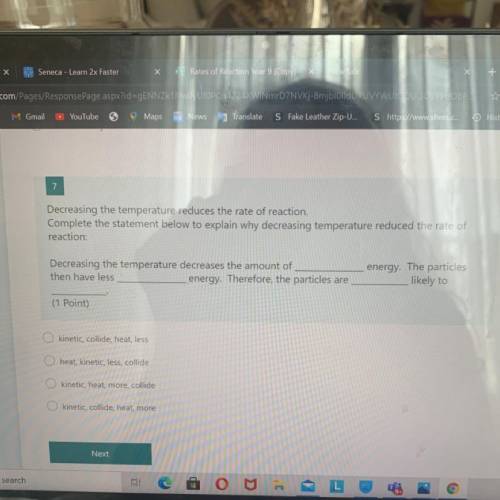
7
Decreasing the temperature reduces the rate of reaction.
Complete the statement below to ex...

Chemistry, 14.04.2021 08:10 jillyan8233
7
Decreasing the temperature reduces the rate of reaction.
Complete the statement below to explain why decreasing temperature reduced the rate of
reaction:
Decreasing the temperature decreases the amount of
then have less
energy. Therefore, the particles are
energy. The particles
likely to
(1 Point)
kinetic, collide, heat, less
heat, kinetic, less, collide
kinetic, heat, more, collide
O kinetic, collide, heat, more
Next

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
You have a sample of a gas that occupies a volume of 17ml at -111 degrees celsius. what volume does the sample occupy at 88 degrees celsius? show all work asap
Answers: 3

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

Chemistry, 22.06.2019 21:30
What is happening when the water inside a kettle heats up and begins to boil
Answers: 1

Chemistry, 22.06.2019 22:00
Choose all the answers that apply. fluorine (f) has an atomic number of 9 and an atomic weight of 18.99. fluorine has a. 9 protons b. 10 neutrons c. 18 electrons d. an atomic mass of 19 e. at least one isotope
Answers: 1
You know the right answer?
Questions



Biology, 25.06.2019 04:30


Biology, 25.06.2019 04:30


Mathematics, 25.06.2019 04:30

Mathematics, 25.06.2019 04:30

Mathematics, 25.06.2019 04:30




Mathematics, 25.06.2019 04:30


History, 25.06.2019 04:30




English, 25.06.2019 04:30




