
Chemistry, 14.04.2021 08:20 zamyapritchard5
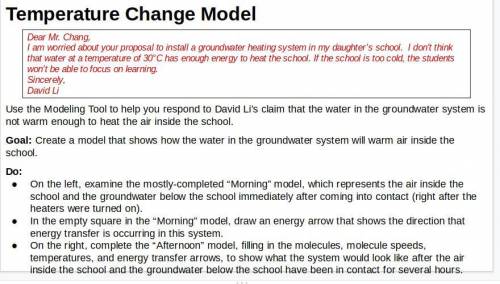
Respond to David Li’s letter. Explain how the groundwater system could heat the air in the school. Explain what would happen to the air temperature at Riverdale School if the groundwater system were used. In addition to the unit vocabulary, be sure to use the terms stability and change in your explanation.



Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:10
Which form of relativism states that people rely on their own standards of right and wrong when making a decision?
Answers: 1

Chemistry, 22.06.2019 03:10
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 18:00
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1
You know the right answer?
Respond to David Li’s letter. Explain how the groundwater system could heat the air in the school....
Questions






English, 20.02.2022 02:00

English, 20.02.2022 02:00



English, 20.02.2022 02:00



Chemistry, 20.02.2022 02:10



Mathematics, 20.02.2022 02:10






