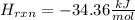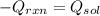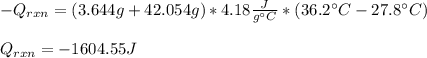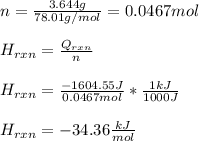Chemistry, 14.04.2021 09:30 ichabella2010
If 3.644 g (78.01 g/mol) is dissolved in 42.054 g of water and the temperature goes
from 27.8°C to 36.2°C, what is the molar Hrxn (kJ/mol)? Assume the solution has s
= 4.18 Hint: what is the mass of the entire solution?
g.°C

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
After cloud droplets form, what must happen to them for precipitation to occur?
Answers: 1

Chemistry, 22.06.2019 00:30
This active feature of earth's crust in building mountain ranges as well as islands. this feature is a a) cavern. b) earthquake. c) mountain. d) volcano.
Answers: 2

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 2

You know the right answer?
If 3.644 g (78.01 g/mol) is dissolved in 42.054 g of water and the temperature goes
from 27.8°C to...
Questions



History, 04.02.2021 20:00






Advanced Placement (AP), 04.02.2021 20:00

Mathematics, 04.02.2021 20:00


Mathematics, 04.02.2021 20:00

Arts, 04.02.2021 20:00

Mathematics, 04.02.2021 20:00


Mathematics, 04.02.2021 20:00



Mathematics, 04.02.2021 20:00