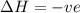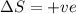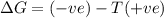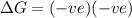Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:30
How does decreasing the gas volume affect the pressure of a gas?
Answers: 1

Chemistry, 21.06.2019 17:40
In the reading, yao chen-yuan describes traveling to deliver a message. why was he willing to risk danger to travelto tientsin? he wanted to the boxers with their cause
Answers: 2

Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1

Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2
You know the right answer?
What does a process require to be spontaneous at all temperatures? answer a catalyst and lower acti...
Questions









Mathematics, 24.03.2020 04:51



Mathematics, 24.03.2020 04:52



English, 24.03.2020 04:52





Mathematics, 24.03.2020 04:53

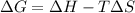
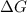 = free energy change
= free energy change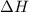 = enthalpy change
= enthalpy change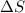 = entropy change
= entropy change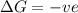 , reaction is spontaneous
, reaction is spontaneous