
Chemistry, 14.04.2021 19:00 gudon986732
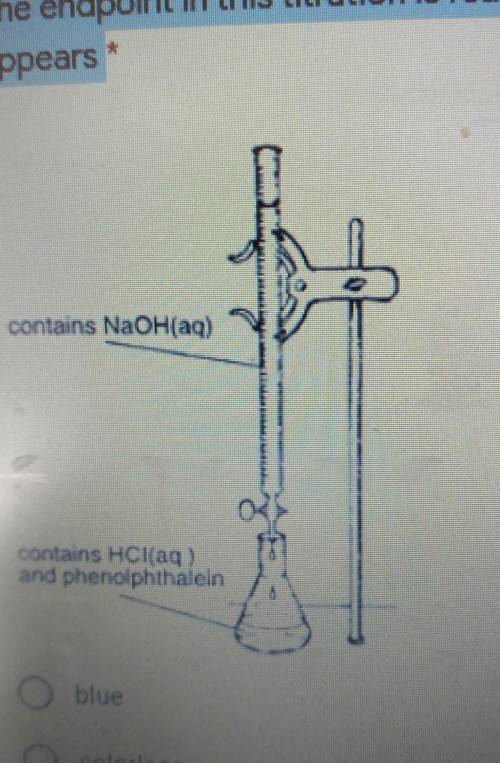
The diagram below shows NaOH(aq) being added to HCl(aq). A few drops 1 of phenolphthalein were added to the flask before the titration was started. The endpoint in this titration is reached when the solution in the flask appears

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:20
Complete the table for ion charge based upon their losing or gaining electrons in the outer shell. (use the periodic table as necessary.) group most likely ionic charge # of valence electrons i +1 ii +2 iii +3 iv +4 or -4 v -3 vi -2 vii -1 viii 0
Answers: 2

Chemistry, 22.06.2019 05:30
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2

Chemistry, 22.06.2019 18:30
How many moles of lead are in 1.50 x 10^12 atoms of lead? could you explain the answer as well and not just give it to me i am refreshing for finals and i need to know how to do it
Answers: 3

Chemistry, 22.06.2019 21:00
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3
You know the right answer?
The diagram below shows NaOH(aq) being added to HCl(aq). A few drops 1 of phenolphthalein were added...
Questions

Mathematics, 06.01.2021 16:40






Mathematics, 06.01.2021 16:40




English, 06.01.2021 16:40

Biology, 06.01.2021 16:40


Physics, 06.01.2021 16:40



Mathematics, 06.01.2021 16:40





