
Chemistry, 15.04.2021 01:20 raffaldarmaki9412
HURRY
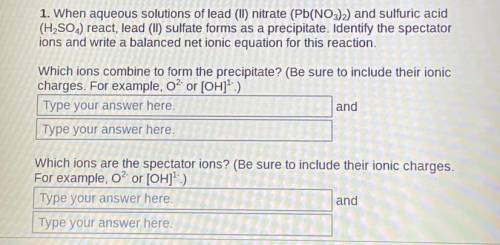
1. When aqueous solutions of lead (II) nitrate (Pb(NO3)2) and sulfuric acid (H2SO4) react, lead (II) sulfate forms as a precipitate. Identify the spectator ions and write a balanced net ionic equation for this reaction.
Which ions combine to form the precipitate? (Be sure to include their ionic charges. For example, O² or [OH]¹-)
2. Which ions are the spectator ions? (Be sure to include their ionic charges. For example, O² or [OH]¹-)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:00
The diagram shows the positions of the sun, moon and earth during spring tides, when the high tides are at their highest and low tides at their lowest. what is it about these positions that causes these high and low tides?
Answers: 3

Chemistry, 22.06.2019 06:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 15:00
Which substance is a steroid? cholesterol fatty acid monosaccharide trans fat
Answers: 1

Chemistry, 22.06.2019 21:00
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2
You know the right answer?
HURRY
1. When aqueous solutions of lead (II) nitrate (Pb(NO3)2) and sulfuric acid (H2SO4) react, le...
Questions

Mathematics, 14.04.2021 17:50


Mathematics, 14.04.2021 17:50

Mathematics, 14.04.2021 17:50

Mathematics, 14.04.2021 17:50

Mathematics, 14.04.2021 17:50

Mathematics, 14.04.2021 17:50


Mathematics, 14.04.2021 17:50

Mathematics, 14.04.2021 17:50


Mathematics, 14.04.2021 17:50


Biology, 14.04.2021 17:50

Social Studies, 14.04.2021 17:50


Geography, 14.04.2021 17:50


Mathematics, 14.04.2021 17:50



