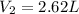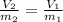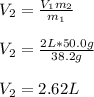Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1

Chemistry, 22.06.2019 15:30
Which of the following are correct values for the ideal gas laws constant r
Answers: 1

Chemistry, 22.06.2019 16:00
How do dying stars contribute to the formation of planets
Answers: 1

You know the right answer?
A 38.2g sample of hydrogen gas, H, is placed into a 2-L soda bottle. IfI keep the pressure and
temp...
Questions





Social Studies, 10.10.2020 20:01

English, 10.10.2020 20:01

World Languages, 10.10.2020 20:01

History, 10.10.2020 20:01

Physics, 10.10.2020 20:01


Mathematics, 10.10.2020 20:01


English, 10.10.2020 20:01



Medicine, 10.10.2020 20:01


History, 10.10.2020 20:01

Mathematics, 10.10.2020 20:01