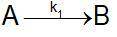Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
What are the similarities of physical and chemical change ?
Answers: 1

Chemistry, 22.06.2019 08:30
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 22.06.2019 18:50
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1

Chemistry, 23.06.2019 01:00
If i had 2 m naoh solution, what does the 2 m stand for? 2 molar, but 2 of a solute in 1
Answers: 1
You know the right answer?
Speed constant data against temperature were obtained for the reaction. Calculate the coefficients a...
Questions


English, 27.04.2021 07:40

Mathematics, 27.04.2021 07:40


Mathematics, 27.04.2021 07:40


Mathematics, 27.04.2021 07:40


Mathematics, 27.04.2021 07:40

History, 27.04.2021 07:40

Mathematics, 27.04.2021 07:40

Mathematics, 27.04.2021 07:40

Mathematics, 27.04.2021 07:40




History, 27.04.2021 07:40


English, 27.04.2021 07:40

Mathematics, 27.04.2021 07:40