Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Apure solvent has a vapor pressure the vapor pressure of a solution. a. equal to b. lower than c. higher than
Answers: 1

Chemistry, 22.06.2019 01:40
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 09:00
What is the percentage composition of carbon in the compound ch4
Answers: 1

Chemistry, 22.06.2019 19:40
What is the wavelength of a 3*10^12 hz infrared wave a 3*10^20m b 1* 10^4m c 3*10^-3m d 1*10^-4 m
Answers: 1
You know the right answer?
Please help me to solve this.
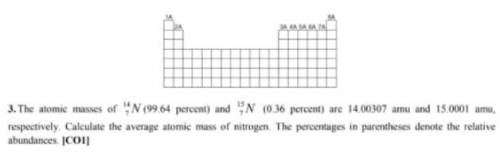
How The atomic musses of 14 N7 (99. 64 percent) and 15N7 (0.36 percen...
Questions


Physics, 03.07.2019 23:00

History, 03.07.2019 23:00





Mathematics, 03.07.2019 23:00

Mathematics, 03.07.2019 23:00

Mathematics, 03.07.2019 23:00



English, 03.07.2019 23:00


Mathematics, 03.07.2019 23:00


Mathematics, 03.07.2019 23:00

Social Studies, 03.07.2019 23:00

Mathematics, 03.07.2019 23:00

History, 03.07.2019 23:00