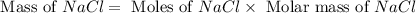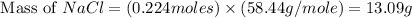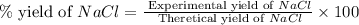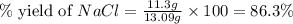Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:10
Amonoprotic acid is an acid that donates a single proton to the solution. suppose you have 0.140 g of a monoprotic acid dissolved in 35.0 ml of water. this solution is then neutralized with 14.5 ml of 0.110 m naoh. what is the molar mass of the acid?
Answers: 1

Chemistry, 21.06.2019 15:00
Theoretically, which metal should be the most reactive? hydrogen lithium francium fluorine
Answers: 1


Chemistry, 22.06.2019 20:30
A40 kilogram skier starts at the top of a 12 meter high slope. at the bottom, she is travelling 10 meters per second. how much energy does she lose to friction
Answers: 2
You know the right answer?
When 15 grams of copper (ii) chloride (cucl2) reacts with 20 grams of sodium nitrate (nano3), 11.3 g...
Questions

Mathematics, 10.03.2020 06:03

Mathematics, 10.03.2020 06:03



Computers and Technology, 10.03.2020 06:03



Mathematics, 10.03.2020 06:03

Mathematics, 10.03.2020 06:03

History, 10.03.2020 06:03

Biology, 10.03.2020 06:03


Physics, 10.03.2020 06:03

Mathematics, 10.03.2020 06:03






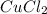 = 15 g
= 15 g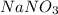 = 20 g
= 20 g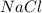 = 58.44 g/mole
= 58.44 g/mole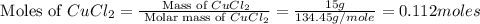
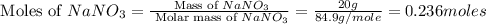
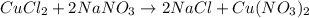
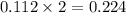 moles of
moles of