
Chemistry, 15.04.2021 23:20 taylormjensen
2. Consider the following chemical equation and equilibrium constant at 25oC:
2COF2(g) <--> CO2(g) + CF4(g) K = 2.2 x 10^6
Calculate the equilibrium constant for the following reaction at 25oC:
2CO2(g) + 2CF4(g) <--> 4COF2
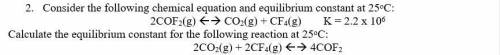

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 08:40
Ageologist determines that a sample of a mineral can't be scratched by a steel nail but can be scratched by a masonry drill bit. based on this information, the sample mineral has to be softer than a. orthoclase. b. fluorite. c. apatite. d. corundum.
Answers: 2

Chemistry, 22.06.2019 14:00
Which of the following is true about a carbonated soft drink? . the carbon dioxide is the solvent, and water is the solute.. the water is the solution, and carbon dioxide is the solvent.. the carbon dioxide is the solution, and the water is the solvent.. the water is the solvent, and the carbon dioxide is the solute.. .
Answers: 1
You know the right answer?
2. Consider the following chemical equation and equilibrium constant at 25oC:
2COF2(g) <--> C...
Questions

Mathematics, 14.05.2021 19:30

Mathematics, 14.05.2021 19:30


Mathematics, 14.05.2021 19:30

Mathematics, 14.05.2021 19:30

History, 14.05.2021 19:30



Mathematics, 14.05.2021 19:30


English, 14.05.2021 19:30

Spanish, 14.05.2021 19:30


Business, 14.05.2021 19:30




Mathematics, 14.05.2021 19:30


Mathematics, 14.05.2021 19:30



