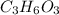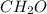Chemistry, 22.01.2020 10:31 bglosson4333
Acompound with the empirical formula ch20 was found to have a molar mass between 89g and 91g. what is the molecular formula of the compound?

Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 01:00
Which of the following is the molecular formula for a simple sugar? a. cooh b. h2o c. oh d. c6h12o6
Answers: 1

Chemistry, 23.06.2019 04:31
What are the coefficients that will balance the skeleton equation below? n2 + h2 → nh3
Answers: 1

Chemistry, 23.06.2019 05:00
Which of the following describes qualitative data? a) recording the temperature of a solid as it is warmed. b) noting the color of a solution as it is heated. c) measuring the volume of an object by water displacement. d) taking the mass of an object using a balance.
Answers: 2

Chemistry, 23.06.2019 07:00
Which of the following statements is true? an atom consists of protons, electrons, and neutrons.an atom consists of protons and neutrons.an atom consists of electrons bonded to one another.an atom consists of protons bonded to one another.
Answers: 1
You know the right answer?
Acompound with the empirical formula ch20 was found to have a molar mass between 89g and 91g. what i...
Questions

Mathematics, 14.12.2020 18:00


History, 14.12.2020 18:00

Mathematics, 14.12.2020 18:00

Mathematics, 14.12.2020 18:00

Chemistry, 14.12.2020 18:00


Mathematics, 14.12.2020 18:00

Arts, 14.12.2020 18:00


Business, 14.12.2020 18:00



Mathematics, 14.12.2020 18:00

Mathematics, 14.12.2020 18:00


Mathematics, 14.12.2020 18:00



Mathematics, 14.12.2020 18:00