
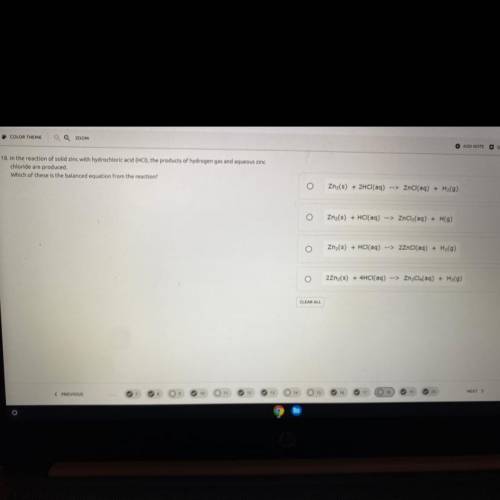
In the reaction of solid zinc with hydrochloric acid (HCI), the products of hydrogen gas and aqueous zinc
chloride are produced.
Which of these is the balanced equation from the reaction?
Zn2(s) + 2HCl(aq) --> ZnCl(aq) + H2(9)
Zn(s) + HCl(aq) --> ZnCl2(aq) + H(9)
Zn (s) + HCl(aq) --> 2ZnCl(aq) + H2(9)
22nz(s) + 4HCl(aq) --> ZnCl4(aq) + H2(9)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 21:40
Tooth enamel consists mainly of the mineral calcium hydroxyapatite, ca_10(po_4)_6(oh)_2. trace elements in teeth of archaeological specimens provide anthropologist with clues about diet and diseases of ancient people. students at hamline university measured strontium in enamel from extracted wisdom teeth by atomic absorption spectroscopy. solutions with a constant total volume of 10.0 ml contained 0.726 mg of dissolved tooth enamel plus variable concentrations of added sr. added sr find the concentration of sr in the 10 ml sample solution in parts per billion = ng/ml. find the concentration of sr in tooth enamel in parts per million = mu g/g.
Answers: 2

Chemistry, 23.06.2019 06:30
(04.01 lc) which of the following is true about science? (5 points) select one: a. it is not influenced by social conditions. b. it is not determined by external local factors. c. political conditions are unable to influence it. d. economic concerns may prevent it from solving problems.
Answers: 1

Chemistry, 23.06.2019 13:30
These traits describe either a chemical or a nuclear reaction. which statements describe a nuclear reaction? check all that apply. involves the loss, gain, or sharing of electrons may involve a change in total mass involve relatively low energy changes occur outside the nucleus involve very high-energy changes involve changes in nuclides when decay occurs
Answers: 1

Chemistry, 23.06.2019 16:00
Afuel has 30.43% nitrogen and 69.57% oxygen. find the molecular formula of the compound if it has a mass of 92 grams per mole a.no b.n2o4 c.no2 d.n4o8
Answers: 1
You know the right answer?
In the reaction of solid zinc with hydrochloric acid (HCI), the products of hydrogen gas and aqueous...
Questions


Mathematics, 04.01.2021 20:00

Chemistry, 04.01.2021 20:00



Mathematics, 04.01.2021 20:00

Chemistry, 04.01.2021 20:00



History, 04.01.2021 20:00

Mathematics, 04.01.2021 20:00




History, 04.01.2021 20:00

Mathematics, 04.01.2021 20:00




Biology, 04.01.2021 20:00



