
Chemistry, 16.04.2021 06:10 endermss1970
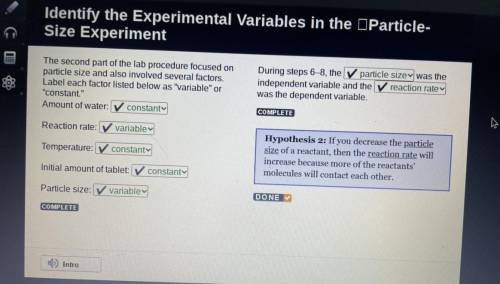
The second part of the lab procedure focused on
particle size and also involved several factors.
Label each factor listed below as "variable" or
"constant."
Amount of water: constant
Reaction rate: variable
Temperature: constanty
Initial amount of tablet: constant
Particle size: variable

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1


Chemistry, 22.06.2019 21:30
The solid xy decomposes into gaseous x and y: xy(s) m x(g) + y(g) kp = 4.1 (at 0 °c) if the reaction is carried out in a 22.4 l container, which initial amounts of x and y will result in the formation of solid xy?
Answers: 1
You know the right answer?
The second part of the lab procedure focused on
particle size and also involved several factors.
Questions

Mathematics, 22.04.2020 21:32


Computers and Technology, 22.04.2020 21:32

Chemistry, 22.04.2020 21:32

Business, 22.04.2020 21:32




Computers and Technology, 22.04.2020 21:32


Mathematics, 22.04.2020 21:32

Mathematics, 22.04.2020 21:32

Mathematics, 22.04.2020 21:32

Biology, 22.04.2020 21:32

Mathematics, 22.04.2020 21:32

History, 22.04.2020 21:32

Mathematics, 22.04.2020 21:32

English, 22.04.2020 21:32





