Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2

You know the right answer?
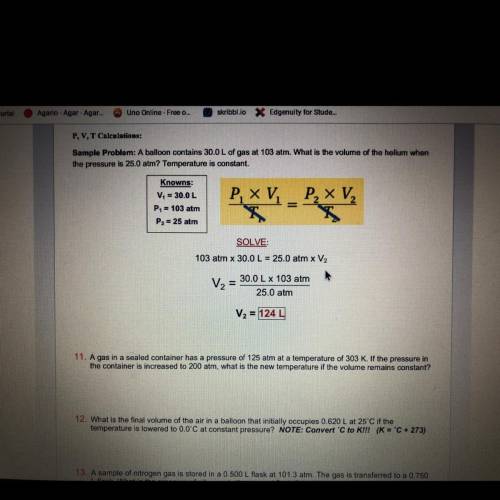
A gas in a sealed container has a pressure of 125 atm at a temperature of 303 K. If the pressure in...
Questions


Mathematics, 15.04.2021 16:00

Mathematics, 15.04.2021 16:00

Mathematics, 15.04.2021 16:00

Mathematics, 15.04.2021 16:00






Mathematics, 15.04.2021 16:00





Social Studies, 15.04.2021 16:00

Mathematics, 15.04.2021 16:00


Mathematics, 15.04.2021 16:00