
Chemistry, 16.04.2021 17:30 SiegeHatake4534
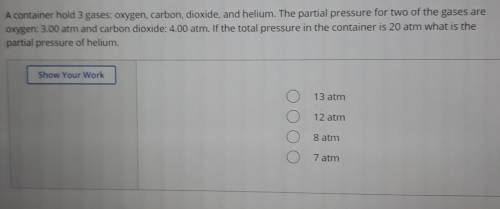
A container hold 3 gases: oxygen, carbon, dioxide, and helium. The partial pressure for two of the gases are oxygen: 3.00 atm and carbon dioxide: 4.00 atm. If the total pressure in the container is 20 atm what is the partial pressure of helium.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Fission of uranium-235 products energy and a. isotopes of smaller elements b. isotopes of larger elements c. lighter isotopes of uranium d. heavier isotopes of uranium
Answers: 3

Chemistry, 22.06.2019 10:00
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1

Chemistry, 22.06.2019 15:30
The identities of substances are the same before and after which type of change
Answers: 1

Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1
You know the right answer?
A container hold 3 gases: oxygen, carbon, dioxide, and helium. The partial pressure for two of the g...
Questions

Geography, 09.07.2019 15:00


Chemistry, 09.07.2019 15:00

Mathematics, 09.07.2019 15:00

History, 09.07.2019 15:00

Chemistry, 09.07.2019 15:00


Social Studies, 09.07.2019 15:00






Biology, 09.07.2019 15:00




History, 09.07.2019 15:00

Mathematics, 09.07.2019 15:00




