
Chemistry, 16.04.2021 18:30 Briannadavis03
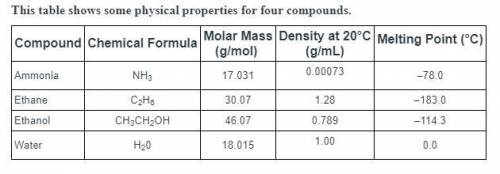
Predict which of these compounds has the highest boiling point.
ammonia, because its low density reduces heat transfer
ammonia, because its low density reduces heat transfer
water, because strong hydrogen bonds form between its molecules
water, because strong hydrogen bonds form between its molecules
ethanol, because its high molecular mass reduces its kinetic energy
ethanol, because its high molecular mass reduces its kinetic energy
ethane, because its low melting point indicates high stability in the liquid phase

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 23:00
What is the formula of the ionic compound composed of calcium cations and chloride anions
Answers: 1

Chemistry, 23.06.2019 12:00
Asubstance that will change shape to fit its container but has a definite volume is in a phase of matter
Answers: 1

Chemistry, 23.06.2019 12:30
An atom holds 7 electrons. use orbital notation to model the probable location of its electrons. an atom hold 22 electrons. use orbital notation to model the probable location of its electrons. an atom holds 17 electrons. use orbital notation to model the probable location of its electrons.
Answers: 1
You know the right answer?
Predict which of these compounds has the highest boiling point.
ammonia, because its low density re...
Questions

Mathematics, 15.01.2023 14:21

Mathematics, 15.01.2023 14:47


Mathematics, 15.01.2023 19:00


Mathematics, 16.01.2023 01:00


Mathematics, 16.01.2023 03:40

Mathematics, 16.01.2023 03:40


History, 16.01.2023 14:00

Biology, 17.01.2023 02:50



Mathematics, 17.01.2023 20:00

Mathematics, 17.01.2023 20:10




History, 18.01.2023 09:00



