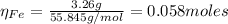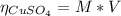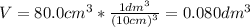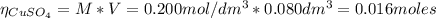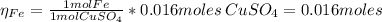Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 11:00
Which statement correctly identifies the scientific question and describes why the question is scientific? question 1 refers to the supernatural.question 2 reflects a moral or social value.question 3 refers to something that can be measured.question 4 reflects a question that can’t be observed.
Answers: 1

Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1

Chemistry, 22.06.2019 13:00
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1
You know the right answer?
2.a. 3.26 g of iron powder are added to 80.0 cm3 of 0.200 mol dm-3 copper(II)
sulfate solution. The...
Questions


Mathematics, 16.10.2020 05:01

English, 16.10.2020 05:01

Social Studies, 16.10.2020 05:01


Mathematics, 16.10.2020 05:01



Mathematics, 16.10.2020 05:01

Biology, 16.10.2020 05:01

Mathematics, 16.10.2020 05:01

Mathematics, 16.10.2020 05:01


Social Studies, 16.10.2020 05:01

History, 16.10.2020 05:01


History, 16.10.2020 05:01



Chemistry, 16.10.2020 05:01

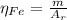
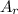 : is the standard atomic weight of iron = 55.845 g/mol
: is the standard atomic weight of iron = 55.845 g/mol