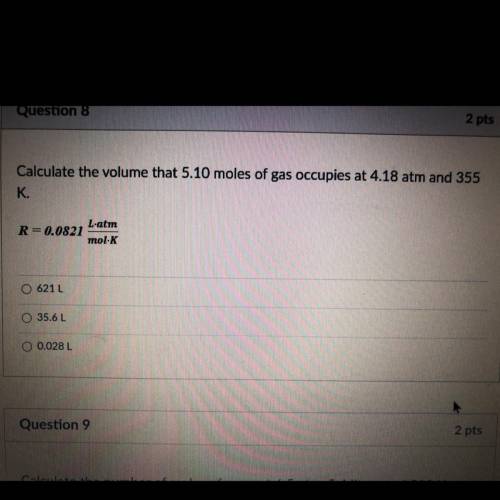
Calculate the volume that 5.10 moles of gas occupies at 4.18 atm and 355 К.
...

Chemistry, 16.04.2021 19:10 KadaLearns
Calculate the volume that 5.10 moles of gas occupies at 4.18 atm and 355 К.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 22.06.2019 13:10
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1

Chemistry, 22.06.2019 14:30
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2
You know the right answer?
Questions




Computers and Technology, 12.08.2020 14:01



Chemistry, 12.08.2020 14:01


Social Studies, 12.08.2020 14:01

Biology, 12.08.2020 14:01

Mathematics, 12.08.2020 14:01

Computers and Technology, 12.08.2020 14:01




Spanish, 12.08.2020 14:01


Biology, 12.08.2020 14:01





