Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
This question is about electrolysis. metal spoons can be coated with silver. this is called electroplating. suggest one reason why spoons are electroplated?
Answers: 1

Chemistry, 22.06.2019 21:00
What is the chemical formula for the compound formed between sodium and flour one
Answers: 1

Chemistry, 22.06.2019 21:30
Isopropyl alcohol, (ch3)2choh, is a common solvent. determine the percent by mass of hydrogen in isopropyl alcohol. a) 6.71% h b) 13.4% h c) 25.0% h d) 53.3% h
Answers: 1

Chemistry, 22.06.2019 22:30
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3
You know the right answer?
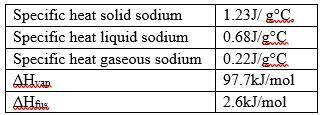
What is the heat required to move 50.0g sodium from a temperature of 300°C to 1100°C and the melting...
Questions

Mathematics, 17.11.2019 08:31


Mathematics, 17.11.2019 08:31


English, 17.11.2019 08:31

Biology, 17.11.2019 08:31



Social Studies, 17.11.2019 08:31

Mathematics, 17.11.2019 08:31

Mathematics, 17.11.2019 08:31

Mathematics, 17.11.2019 08:31


Biology, 17.11.2019 08:31



Advanced Placement (AP), 17.11.2019 08:31

Physics, 17.11.2019 08:31


Chemistry, 17.11.2019 08:31