
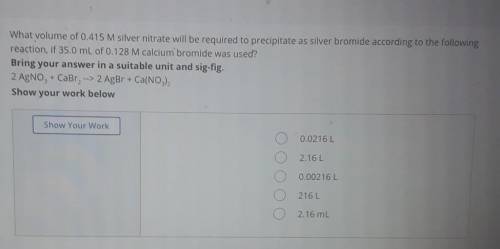
What volume of 0.415 M silver nitrate will be required to precipitate as silver bromide according to the following reaction, if 35.0 mL of 0.128 M calcium bromide was used? Bring your answer in a suitable unit and sig-fig. 2 AgNO3 + CaBr2 --> 2 AgBr + Ca(NO3)2 Show your work below Show Your Work 0.0216 L 2.16 L 0.00216L 216L 2.16 mL

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 22.06.2019 07:50
Which of the following electromagnetic waves can create ions?
Answers: 2


You know the right answer?
What volume of 0.415 M silver nitrate will be required to precipitate as silver bromide according to...
Questions


Computers and Technology, 27.01.2021 04:30


Mathematics, 27.01.2021 04:30

Arts, 27.01.2021 04:30

Chemistry, 27.01.2021 04:30




Mathematics, 27.01.2021 04:30

Mathematics, 27.01.2021 04:30

Mathematics, 27.01.2021 04:30

Arts, 27.01.2021 04:30

History, 27.01.2021 04:30


Biology, 27.01.2021 04:30

Mathematics, 27.01.2021 04:30


Biology, 27.01.2021 04:30

Mathematics, 27.01.2021 04:30



