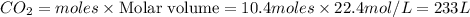Chemistry, 17.04.2021 01:40 npellot123
2C4H10+13O2-->8CO2+10H2O Using the predicted and balanced equation, How many Liters of CO2 can be produced from 150 grams of C4H10?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
City a and city b had two different temperatures on a particular day. on that day, four times the temperature of city a was 8â° c more than 3 times the temperature of city b. the temperature of city a minus twice the temperature of city b was â’3â° c. what was the temperature of city a and city b on that day? city a was 5â° c, and city b was 4â° c. city a was 3â° c, and city b was â’1â° c. city a was 8â° c, and city b was â’3â° c. city a was 5â° c, and city b was â’5â° c.
Answers: 2

Chemistry, 22.06.2019 17:30
What type of organic molecule comprises the majority of a potato?
Answers: 1

Chemistry, 23.06.2019 03:00
What volume does 1.70 ×10–3 mol of chlorine gas occupy if its temperature is 20.2 °c and its pressure is 795 mm hg?
Answers: 3

Chemistry, 23.06.2019 03:00
Achemical equilibrium between gaseous reactants and products is shown. n2(g) + 3h2(g) ⇌ 2nh3(g) how will the reaction be affected if the pressure on the system is increased? it will shift toward the reactant side as there is lower pressure on the reactant side. it will shift toward the product side as there is higher pressure on the product side. it will shift toward the reactant side as there are a greater number of moles of gas on the reactant side. it will shift toward the product side as there are a fewer number of moles of gas on the product side.
Answers: 2
You know the right answer?
2C4H10+13O2-->8CO2+10H2O Using the predicted and balanced equation, How many Liters of CO2 can be...
Questions

Mathematics, 24.12.2020 01:30

History, 24.12.2020 01:30

Mathematics, 24.12.2020 01:30




Arts, 24.12.2020 01:30






Mathematics, 24.12.2020 01:30

Mathematics, 24.12.2020 01:30

Physics, 24.12.2020 01:30

Biology, 24.12.2020 01:30



Physics, 24.12.2020 01:40

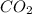 will be produced from 150 grams of
will be produced from 150 grams of 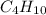
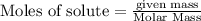
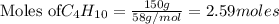
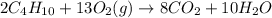
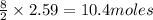 of
of