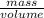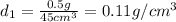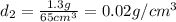Ascientist compares two samples of white powder. one powder was present at the beginning of an experiment. the other powder was present at the end. she wants to determine whether a chemical reaction has occurred. she finds that neither sample bubbles or dissolves in water. she measures the mass and volume of the solids. sample one has a volume of 45 cm3 and a mass of 0.5
g. sample two has a volume of 65 cm3 and a mass of 1.3
g. what should the scientist conclude?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 21:20
One way in which the useful metal copper is produced is by dissolving the mineral azurite, which contains copper(ii) carbonate, in concentrated sulfuric acid. the sulfuric acid reacts with the copper(ii) carbonate to produce a blue solution of copper(ii) sulfate. scrap iron is then added to this solution, and pure copper metal precipitates out because of the following chemical reaction: (s) (aq) (s) (aq) suppose an industrial quality-control chemist analyzes a sample from a copper processing plant in the following way. he adds powdered iron to a copper(ii) sulfate sample from the plant until no more copper will precipitate. he then washes, dries, and weighs the precipitate, and finds that it has a mass of .
Answers: 2

Chemistry, 22.06.2019 22:20
How do cfcs cause ozone depletion? how do cfcs cause ozone depletion? ultraviolet radiation breaks down cfcs, molecules containing chlorine. chlorine then breaks one oxygen atom away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation breaks down cfcs, molecules containing chlorine. chlorine then breaks two oxygen atoms away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation creates cfcs, molecules containing chlorine. chlorine then breaks two oxygen atoms away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation creates cfcs, molecules containing chlorine. chlorine then breaks one oxygen atom away from ozone, leaving behind a paired oxygen molecule.
Answers: 2

Chemistry, 23.06.2019 00:00
Predict the relative bond lengths of the three carbon-oxygen bonds in the carbonate ion (co2−3). what would you expect the charge to be on each oxygen? match the words in the left column to the appropriate blanks in the sentences on the right. make certain each sentence is complete before submitting your answer.
Answers: 3

Chemistry, 23.06.2019 00:00
Before it was launched, a helium-filled balloon had a pressure of 201 kpa at a temperature of 27°c. at an altitude of 15,000 m, the pressure had decreased to 2.5 kpa and the temperature had dropped to -14 °c. the volume of the balloon increased to 59.3 m3. what is the original volume of the balloon? 13 m3 0.85 m3 0.077 m3 1.17 m3
Answers: 3
You know the right answer?
Ascientist compares two samples of white powder. one powder was present at the beginning of an exper...
Questions



Mathematics, 22.09.2021 02:10

Mathematics, 22.09.2021 02:10


Mathematics, 22.09.2021 02:10

Mathematics, 22.09.2021 02:10

English, 22.09.2021 02:10


English, 22.09.2021 02:10

History, 22.09.2021 02:10

Geography, 22.09.2021 02:10




Mathematics, 22.09.2021 02:10



Mathematics, 22.09.2021 02:10