
Chemistry, 17.04.2021 19:40 maddy3lizabeth
Explain why each of these elements occur as diatomic molecules, Match the words in the left column to the appropriate blanks in the sentences on the right.
triple
double
octet
single
duet
When two hydrogen atoms pair together, they form a bond to achieve a complete which is a stable configuration.
When two halogens poir together, they form abond to achieve a complete which is a stable configuration
to achieve a completo
When two oxygen atoma pair together, they form abond to achieve a complete which is a stable configuration
When two nitrogen atomo poir together, they form abond to achieve a complete which is a stable configuration

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
A100-watt light bulb radiates energy at a rate of 100 j/s. (the watt, a unit of power or energy over time, is defined as 1 j/s.) if all of the light emitted has a wavelength of 525 nm , how many photons are emitted per second?
Answers: 1

Chemistry, 22.06.2019 14:40
Pastoral farming is best described as a. a method of raising livestock and moving herds b. an african method of agriculture c. a method of cultivating crops on poor soils d. a common method of desert farming select the best answer from the choices provided a b c d
Answers: 2

Chemistry, 23.06.2019 06:00
If you try to move a piano and are unable to move it, did you perform any work in the scientific sense of the word? yes? or no? this question is worth 20 points! let it be correct!
Answers: 1

Chemistry, 23.06.2019 14:30
Will give imagine you are given a mystery element. it is, however, a discovered and known element. you may perform a maximum of two observations or tests to determine its identity. time and money is critical, so you need to prioritize your tests. if you can get by with a single test, you get 100 super-geek points from your research lab team. pick your two tests, number them as #1 and #2, and justify why you think these two will certainly be enough (and why the first might well be enough all by itself.) the available tests are classification into metal, non-metal, or metalloid, count of valence electrons, count of electron shells, atomic radius (error range: +/- 1 pm), electronegativity (error range: +/- 0.1), first ionization energy (error range: +/- 10 kj/mole), melting point (error range: +/- 10 c), and boiling point (error range: +/- 20 c).
Answers: 2
You know the right answer?
Explain why each of these elements occur as diatomic molecules, Match the words in the left column t...
Questions

English, 28.01.2021 22:10

World Languages, 28.01.2021 22:10





Mathematics, 28.01.2021 22:10


Mathematics, 28.01.2021 22:10



Biology, 28.01.2021 22:20



Biology, 28.01.2021 22:20

History, 28.01.2021 22:20

Mathematics, 28.01.2021 22:20


Chemistry, 28.01.2021 22:20

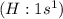 has one valence electron each, so sharing of electrons result in formation of single bond and the same electron configuration as the Helium and thus complete its duet.
has one valence electron each, so sharing of electrons result in formation of single bond and the same electron configuration as the Helium and thus complete its duet.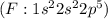 have 7 valence electron each, so sharing of electrons result in formation of single bond. The stable noble gas configuration is attained and thus complete its octet.
have 7 valence electron each, so sharing of electrons result in formation of single bond. The stable noble gas configuration is attained and thus complete its octet.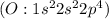 has six valence electrons each, so sharing of two electrons result in a double bond. The stable noble gas configuration is attained and thus complete its octet.
has six valence electrons each, so sharing of two electrons result in a double bond. The stable noble gas configuration is attained and thus complete its octet.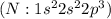 has 5 valence electrons, so sharing of three electrons result in a triple bond. The stable noble gas configuration is attained and thus complete its duet.
has 5 valence electrons, so sharing of three electrons result in a triple bond. The stable noble gas configuration is attained and thus complete its duet.


