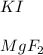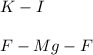Chemistry, 18.04.2021 01:30 SophieStar15
Use the Lewis model to determine the formula for the compound that forms from each pair of atoms.
Express your answer as a chemical formula.
1) K and I
2) Mg and F

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:50
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 22.06.2019 15:30
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3


Chemistry, 22.06.2019 20:30
Citric acid has a ph between 1 and 3. it is considered to be aa. weak acidb. weak basec. strong based. strong acid
Answers: 2
You know the right answer?
Use the Lewis model to determine the formula for the compound that forms from each pair of atoms.
E...
Questions


English, 13.05.2021 18:30

Physics, 13.05.2021 18:30


Mathematics, 13.05.2021 18:30


Biology, 13.05.2021 18:30

Mathematics, 13.05.2021 18:40


Social Studies, 13.05.2021 18:40

Mathematics, 13.05.2021 18:40


Mathematics, 13.05.2021 18:40

Mathematics, 13.05.2021 18:40



Mathematics, 13.05.2021 18:40



Mathematics, 13.05.2021 18:40