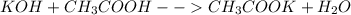Chemistry, 18.04.2021 02:10 firenation18
A 15.5 mL sample of 0.215 M KOH solution required 21.2 mL of aqueous acetic acid solution in a titration experiment. Calculate the molarity of the acetic acid solution.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1


Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3

Chemistry, 22.06.2019 23:00
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3
You know the right answer?
A 15.5 mL sample of 0.215 M KOH solution required 21.2 mL of aqueous acetic acid solution in a titra...
Questions


History, 01.03.2021 02:40




Mathematics, 01.03.2021 02:40


Spanish, 01.03.2021 02:40

Mathematics, 01.03.2021 02:50

Mathematics, 01.03.2021 02:50

Spanish, 01.03.2021 02:50


Mathematics, 01.03.2021 02:50





Mathematics, 01.03.2021 02:50

Arts, 01.03.2021 02:50

Mathematics, 01.03.2021 02:50