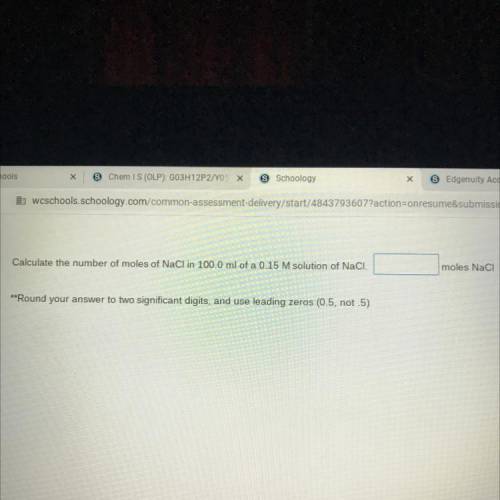
Calculate the number of moles of NaCl in 100.0 ml of a 0.15 M solution of NaCl.
...

Chemistry, 18.04.2021 03:30 ginger87771
Calculate the number of moles of NaCl in 100.0 ml of a 0.15 M solution of NaCl.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:00
Note the ph and poh values labeled with letters on the ph scale below. based on log rules and the way ph is calculated, what is the difference in [oh– ] concentration between point a and point b. a) 10^1 b) 10^5 c) 10^6 d) 10^7
Answers: 1

Chemistry, 22.06.2019 08:00
If 90.0 grams of ethane reacted with excess chlorine,how many grams of dicarbon hexachloride would form
Answers: 1

Chemistry, 22.06.2019 09:30
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3

Chemistry, 22.06.2019 15:30
The gulf stream is a warm water current that flows away from the equator to northern europe. witch of these does it cause. a. crashes of warm and cool water in the ocean b.colder climates near the equator c.large waves on the cost of europe d.warm climates in northern europe
Answers: 1
You know the right answer?
Questions




Mathematics, 20.03.2020 23:58

Mathematics, 20.03.2020 23:58

Mathematics, 20.03.2020 23:58




Mathematics, 20.03.2020 23:58





Biology, 20.03.2020 23:58


Mathematics, 20.03.2020 23:58



History, 20.03.2020 23:58



