
Chemistry, 18.04.2021 07:50 alyssakerr17
How many moles of argon gas would be present in a 37.0 liter vessel at 45.00 °C at a pressure of 2.50 atm?
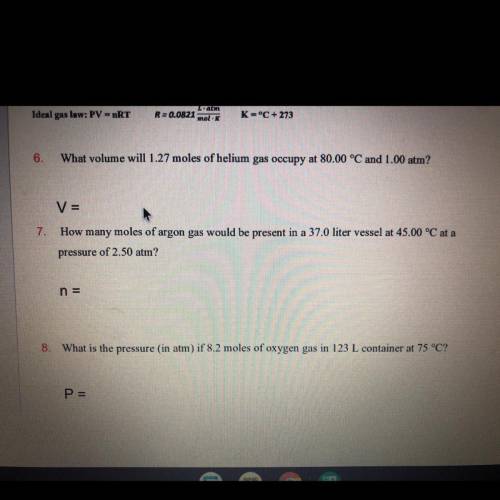

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 15:30
Why does earth rotate? because earth is formed from cold gases collapsing due to gravity because the matter in the nebula that formed earth was spinning because earth forms more than 99% of the mass of the solar system because the hydrogen atoms inside the nebula fused to form helium
Answers: 1

Chemistry, 23.06.2019 01:00
If a straight-chain hydrocarbon is a gas at room temperature, how many carbon atoms will it have? a. 6 carbon atoms b. 12 carbon atoms c. 24 carbon atoms d. 3 carbon atoms
Answers: 1

Chemistry, 23.06.2019 03:00
What volume does 1.70 ×10–3 mol of chlorine gas occupy if its temperature is 20.2 °c and its pressure is 795 mm hg?
Answers: 3

Chemistry, 23.06.2019 06:30
The molar mass of cu is 63.55 g/mol. the number of grams of cu produced in this reaction is
Answers: 3
You know the right answer?
How many moles of argon gas would be present in a 37.0 liter vessel at 45.00 °C at a pressure of 2.5...
Questions



History, 23.10.2019 18:20

English, 23.10.2019 18:20

Biology, 23.10.2019 18:20

History, 23.10.2019 18:20

Mathematics, 23.10.2019 18:20


Mathematics, 23.10.2019 18:20

History, 23.10.2019 18:20



Chemistry, 23.10.2019 18:20

Physics, 23.10.2019 18:20

History, 23.10.2019 18:20





Health, 23.10.2019 18:20



