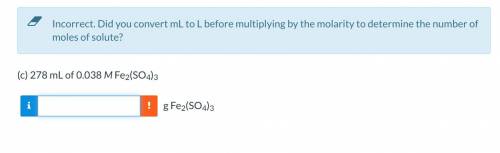
Calculate the grams of solute in each of the following solution: 278 mL of 0.038 M Fe2(SO4)3
...

Chemistry, 18.04.2021 14:00 20guadalupee73248
Calculate the grams of solute in each of the following solution: 278 mL of 0.038 M Fe2(SO4)3

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:00
Which of the following happens during cell division? (a) energy is created (b) waste is eliminated (c) carbon dioxide is released (d) damaged cells are replaced
Answers: 1

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2

Chemistry, 23.06.2019 06:00
Each step in the following process has a yield of 70% ch4 + 4cl2 yield ccl4 +4hcl ccl4 + 2hf yield ccl2f2 + 2hcl of 4.50 mole ch4 reacts what is the total amount of hcl produced
Answers: 3

Chemistry, 23.06.2019 08:00
Drag each pressure unit with the corresponding number to describe standard atmospheric pressure
Answers: 1
You know the right answer?
Questions



Spanish, 18.03.2021 23:50



Mathematics, 18.03.2021 23:50

Social Studies, 18.03.2021 23:50



Mathematics, 18.03.2021 23:50

Mathematics, 18.03.2021 23:50

Mathematics, 18.03.2021 23:50






Mathematics, 18.03.2021 23:50

Mathematics, 18.03.2021 23:50

Mathematics, 18.03.2021 23:50



