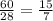Silicon can be made by heating a mixture of sand (silicon dioxide) with magnesium
powder.
The...

Silicon can be made by heating a mixture of sand (silicon dioxide) with magnesium
powder.
The equation for this reaction is shown below.
SiO2 (s)+ 2Mg (s) → 2MgO (s) + Si (s)
Calculate the mass of silicon dioxide needed to make 1 g of silicon.
Relative atomic masses: O = 16; Si = 28
Mass = ?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1

Chemistry, 22.06.2019 23:00
What is the oxidation state of each individual carbon atom in c2o42−?
Answers: 1

Chemistry, 22.06.2019 23:00
How does the value of the equilibrium constant show that a reaction reaches equilibrium very quickly? (a) the equilibrium constant is large. (b) the equilibrium constant is small. (c) the equilibrium constant is zero. (d) the value of the equilibrium constant does not show how quickly a reaction comes to equilibrium.
Answers: 1
You know the right answer?
Questions

Mathematics, 10.05.2021 05:50

Health, 10.05.2021 05:50





Mathematics, 10.05.2021 05:50




Mathematics, 10.05.2021 05:50

Mathematics, 10.05.2021 05:50

Social Studies, 10.05.2021 05:50


Mathematics, 10.05.2021 05:50



Social Studies, 10.05.2021 05:50