S8 +O2 = SO8
Given 80 mols of product, find the amount of each reactant in mols....

Chemistry, 19.04.2021 06:00 AdoreeLayla
S8 +O2 = SO8
Given 80 mols of product, find the amount of each reactant in mols.

Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 00:30
Ok, so i have 2 questions. try to answer them both: (the topic is fire) 1) how can you represent the chemical reaction of fire? 2) what kind of bond is formed in this chemical reaction
Answers: 3

Chemistry, 23.06.2019 02:00
What causes the appearance of lines in a emission spectrum
Answers: 1

Chemistry, 23.06.2019 02:30
Asubstance is held in an open container. its particles move past one another at random speeds but do not leave the container. heat is removed from the system, and the particles slow down. when enough heat is removed, the particles no longer have enough speed to overcome the weak attractive forces between them. when this happens, the substance enters its solid state. the process described above is known as .
Answers: 3

Chemistry, 23.06.2019 07:00
An unknown substance is a white solid at room temperature and has a melting point of 78 °c. which of the following substances is most likely to be the identity of the unknown sample? a. naphthalene, a molecular solid with the formula c10h8 b. silica, a network solid held together by covalent bonds with the formula sio2 c. calcium chloride, an ionic compound with the formula cacl2 d. water, an molecular compound with the formula h2o
Answers: 2
You know the right answer?
Questions





Mathematics, 12.06.2020 22:57

Mathematics, 12.06.2020 22:57




Mathematics, 12.06.2020 23:57

Mathematics, 12.06.2020 23:57


Mathematics, 12.06.2020 23:57



English, 12.06.2020 23:57

Mathematics, 12.06.2020 23:57



Mathematics, 12.06.2020 23:57

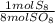 = 10 mol S₈80 mol SO₈ *
= 10 mol S₈80 mol SO₈ * 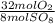 = 320 mol O₂
= 320 mol O₂

