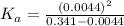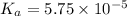Chemistry, 19.04.2021 16:10 NarutoBeast8049
In the laboratory, a general chemistry student measured the pH of a 0.341 M aqueous solution of benzoic acid, C6H5COOH to be 2.351. Use the information she obtained to determine the Ka for this acid.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 22.06.2019 21:20
Phosgene (carbonyl chloride), cocl2, is an extremely toxic gas that is used in manufacturing certain dyes and plastics. phosgene can be produced by reacting carbon monoxide and chlorine gas at high temperatures: co(g) cl2(g)⇌cocl2(g) carbon monoxide and chlorine gas are allowed to react in a sealed vessel at 477 ∘c . at equilibrium, the concentrations were measured and the following results obtained: gas partial pressure (atm) co 0.830 cl2 1.30 cocl2 0.220 what is the equilibrium constant, kp, of this reaction
Answers: 2

Chemistry, 22.06.2019 23:00
What is the average rate of the reaction between 10 and 20 s?
Answers: 1

You know the right answer?
In the laboratory, a general chemistry student measured the pH of a 0.341 M aqueous solution of benz...
Questions

Mathematics, 04.08.2020 15:01

Mathematics, 04.08.2020 15:01

Mathematics, 04.08.2020 15:01

Social Studies, 04.08.2020 15:01



English, 04.08.2020 15:01



English, 04.08.2020 15:01

History, 04.08.2020 15:01

Mathematics, 04.08.2020 15:01

Biology, 04.08.2020 15:01

History, 04.08.2020 15:01

Mathematics, 04.08.2020 15:01

Mathematics, 04.08.2020 15:01

Computers and Technology, 04.08.2020 15:01

Mathematics, 04.08.2020 15:01


Business, 04.08.2020 15:01

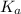 for the acid is
for the acid is 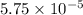
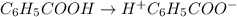
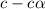
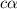
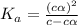
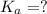
![pH=-log[H^+]](/tpl/images/1268/1073/15713.png)
![[H^+]=10^{-2.351}=0.0044](/tpl/images/1268/1073/85ea6.png)
![[H^]=c\alpha=0.0044](/tpl/images/1268/1073/4f06a.png)