Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Covalent network solids typically have melting points and boiling points. the chemical formula of a network solid indicates in the molecule.
Answers: 3

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1

Chemistry, 22.06.2019 13:00
One of the hopes for solving the world's energy problem is to make use of the fusion reaction 21h +31h --> 42he + 10n + energy how much energy is released when 1 mol of deuterium is fused with 1 mol of tritium according to the above reaction? the masses of the atoms and the neutrons are as follows: 21h = 2.0140 amu 31h = 3.01605 amu 42he = 4.002603 amu 10n = 1.008665 amu. the speed of light is 2.9979 x 108 m/s.
Answers: 1

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1
You know the right answer?
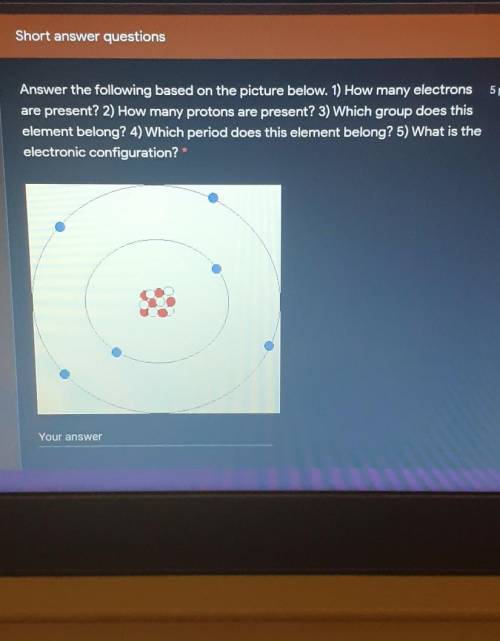
Answer the following based on the picture below. 1) How many electrons
are present? 2) How many pro...
Questions

History, 23.11.2020 18:10

Health, 23.11.2020 18:10



Mathematics, 23.11.2020 18:10




Health, 23.11.2020 18:10

Mathematics, 23.11.2020 18:10

Health, 23.11.2020 18:10



Mathematics, 23.11.2020 18:10



Mathematics, 23.11.2020 18:10

Mathematics, 23.11.2020 18:10

Arts, 23.11.2020 18:10