

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 01:50
7. what temperature is need to just dissolve 50 g of nh4cl in 75 g of water? '
Answers: 1

Chemistry, 22.06.2019 09:40
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1
You know the right answer?
Consider the reaction, C2H4(g) + H2(g) - C2H6(8), where AH = -137 kJ. How many kilojoules are releas...
Questions

Mathematics, 14.07.2020 20:01

History, 14.07.2020 20:01





Mathematics, 14.07.2020 20:01

Social Studies, 14.07.2020 20:01

Mathematics, 14.07.2020 20:01

Chemistry, 14.07.2020 20:01



Mathematics, 14.07.2020 20:01

Computers and Technology, 14.07.2020 20:01

History, 14.07.2020 20:01



Mathematics, 14.07.2020 20:01


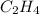 reacts.
reacts.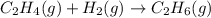

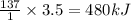 of energy
of energy


