
Chemistry, 19.04.2021 17:50 uehlingt39
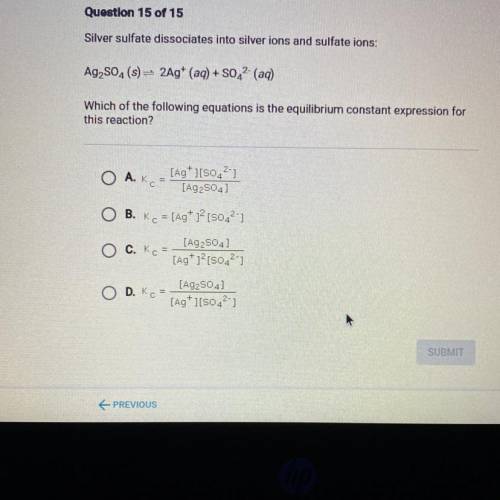
Silver sulfate dissociates into silver ions and sulfate ions:
Ag2SO4(s) = 2Ag+ (aq) + SO42- (aq)
Which of the following equations is the equilibrium constant expression for
this reaction?
A. Kot
[Ag+][80.21
[Ag 2504)
O B. Ko = [Ag+ 12 [5042")
[A92504)
O C. KE =
[Ag+1?[s042-7
O D. KG
[A92504
[Ag+ ][5042-)

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 14:00
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2

Chemistry, 22.06.2019 16:00
Answer asap : ( a. how does mucus prevent the entry of pathogens? b. describe two ways white blood cells protect us from pathogens.
Answers: 1

You know the right answer?
Silver sulfate dissociates into silver ions and sulfate ions:
Ag2SO4(s) = 2Ag+ (aq) + SO42- (aq)
Questions




English, 26.02.2020 03:27






Mathematics, 26.02.2020 03:27


Computers and Technology, 26.02.2020 03:27










